Zeroth Law of Thermodynamics
Zeroth Law of Thermodynamics: Overview
This topic covers concepts, such as, Thermal Equilibrium, Zeroth Law of Thermodynamics, Statement of Zeroth Law of Thermodynamics, Heat Transfer from Hot to Cold & Concept of Temperature etc.
Important Questions on Zeroth Law of Thermodynamics
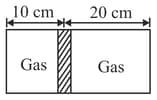
Diagram shows a horizontal cylindrical container of length , which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in the diagram. The temperature of left part of cylinder is and that on right part is . Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator after a long when gases on the two parts of cylinder are in thermal equilibrium.
Explain the relation of kinetic energy with the absolute temperature.
Kinetic energy is inversely related to the absolute temperature of a body.
Particles of a cold body will have higher kinetic energy the particles of a hot body.
Absolute temperature of a body is proportional to the _____ energy of the body.
Which body will have the highest kinetic energy: Cold body or hot body?
Thermal equilibrium between systems is a transitive relation. Which law of thermodynamics supports this? Explain.
Which law of thermodynamics accounts for thermal equilibrium?
If two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other. This is stated by _____ (first/zeroth) law of thermodynamics.
Zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
Zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third, then they may or may not be in thermal equilibrium with each other.
Which of the following statements is correct?
Explain the direction of heat transfer between hot body and cold body.
Heat flows from hot to cold objects. This can be explained by _____ (zeroth/first) law of thermodynamics.
Which law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
Describe about the law which states that thermal equilibrium between systems is a transitive relation.
_____ (Zeroth/First) law states that if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
First law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
State the zeroth law of thermodynamics.
The circuit below is used to heat water kept in a bucket.
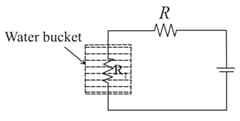
Assuming heat loss only by Newton's law of cooling, the variation in the temperature of the water in the bucket as a function of time is depicted by
